
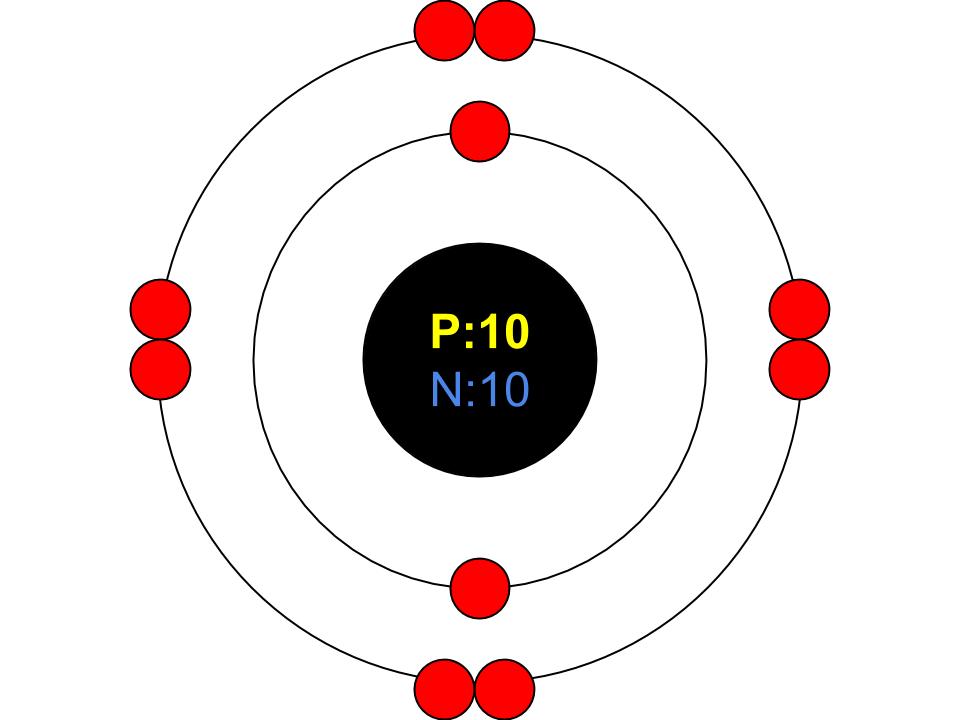
The atom then remains in its lowest-energy state, which is also called the ground state. The amount of energy supplied is not absorbed by the atom or by the electrons. Since there are no energy states between two shells, at lower energy levels, no electron can be brought to a next higher shell. The absorption of energy can only happen if the energy supply is at least as large as an electron can be “lifted” from its current shell to the next higher one. Such an energy intake is also referred to as absorption. With Bohr’s shell model can now finally be understood why atoms absorb only certain amounts of energy. This is the first innovation in the atomic concept that Bohr formulated as a postulate:Įlectrons move only on discrete shells around the nucleus, each representing a certain energy level! Figure: Atomic model of Bohr (shell model)Ī postulate is a principle on which a theory is based!

The further away the shell is from the atomic nucleus, the more energetic is the state of an electron located there (higher energy level). An electron can not assume an energy state that lies between two shells, since there can not be any electron there. For this reason, the Bohr model is also referred to as shell model.Įach shell corresponds to a specific energy value of the electron (also called energy state or energy level). He postulated that the electrons can only move in certain orbits around the atomic nucleus, comparable to the planetary motion around the sun. Therefore, he expanded the atom model of Rutherford especially with regard to the atomic shell. The physicist Niels Bohr suspected that this behavior must have something to do with the electron shell. If the energy is only slightly lower, suddenly there is no illumination (for example Franck-Hertz experiment). For example, some atoms can only be excited to glow when bombarded with particles of specific energy. However, some phenomena can not be explained with this atomic model. The Rutherford model in many cases provides a very good explanation of physical processes in matter. According to the Bohr’s atomic model, electrons move on discrete shells around the nucleus (discrete energy levels).


 0 kommentar(er)
0 kommentar(er)
